
This light-producing process, called fluorescence, causes a glow in the region where the ions emerge from the cathode.Īn anode ray ion source typically is an anode coated with the halide salt of an alkali or alkaline earth metal. In returning to their former energy levels these atoms or molecules release the energy that they had gained. These are the anode rays.īy the time they reach the cathode, the ions have been accelerated to a sufficient speed such that when they collide with other atoms or molecules in the gas they excite the species to a higher energy level. The positive ions are all attracted to the negative cathode, and some pass through the holes in the cathode. These ions and electrons in turn strike more atoms, creating more positive ions in a chain reaction. These collide with atoms of the gas, knocking electrons off of them and creating more positive ions.

When the high voltage is applied to the tube, its electric field accelerates the small number of ions (electrically charged atoms) always present in the gas, created by natural processes such as radioactivity. The process by which anode rays are formed in a gas-discharge anode ray tube is as follows. Goldstein called these positive rays Kanalstrahlen, "channel rays", or "canal rays", because they were produced by the holes or channels in the cathode.

These rays are beams of particles moving in a direction opposite to the " cathode rays", which are streams of electrons which move toward the anode. When an electrical potential of several thousand volts is applied between the cathode and anode, faint luminous "rays" are seen extending from the holes in the back of the cathode.
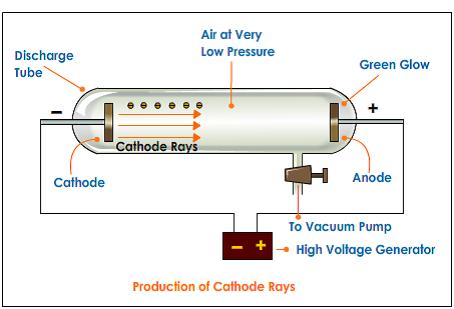
Goldstein used a gas-discharge tube which had a perforated cathode.


 0 kommentar(er)
0 kommentar(er)
